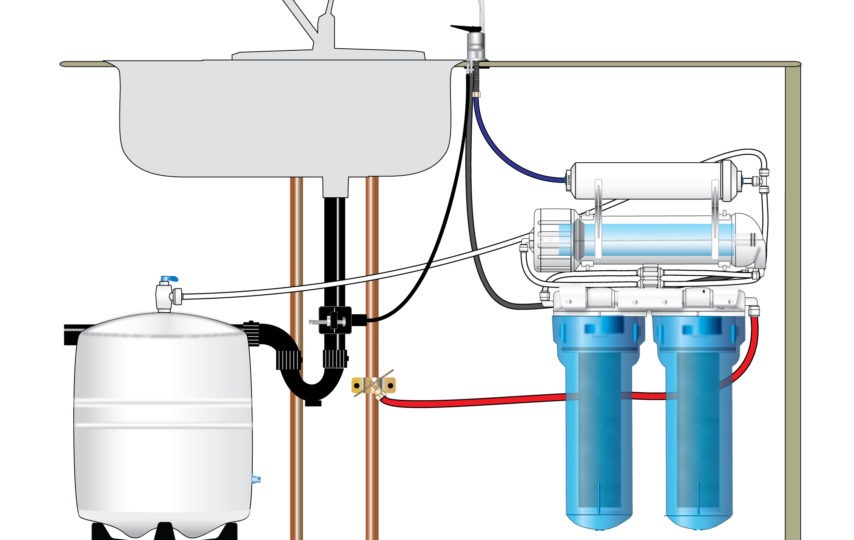
Reverse Osmosis commonly known as RO water purifier system is a technology that is used to eliminate a huge majority of contaminants from water by pushing the water under pressure via a semi-permeable membrane. This is the procedure wherein the water is demineralized or deionized.
To understand the process and purpose of Reverse Osmosis you must first know the process of Osmosis. Osmosis is a naturally occurring incident and one of the most vital processes. It is a procedure where a weaker saline solution will travel to a strong saline solution. The instances of osmosis are when the kidneys absorb water from blood and plant roots absorb water from the soil.
Reverse Osmosis is the procedure of Osmosis in overturn. While Osmosis occurs naturally without the necessity of energy, to reverse the procedure of osmosis energy is required. A reverse osmosis membrane is a semi-permeable membrane that enables the course of water molecules but not the common organics, dissolved salts, pyrogens and bacteria. But the water need to be pushed through the reverse osmosis membrane by applying pressure that is more than the naturally occurring osmotic pressure so as to deionize or demineralize water in the procedure, enabling pure water through while holding back a majority of pollutants.
How RO works?
RO works by using a high pressure pump to boost the pressure on the salt side of the RO and compel the water across the semi-permeable RO membrane, leaving almost all dissolved salts behind in the reject stream. The amount of pressure necessary depends on the salt concentration of the feed water. The more concentrated the feed water, the more pressure is needed to prevail over the osmotic pressure.
The deionized or demineralized water is called permeate water. The water flow that carries the concentrated contaminants which did not pass through the RO membrane is called the reject stream. As the feed water enters the RO membrane under pressure the water molecules pass through the semi-permeable membrane and the contaminants and salts are not allowed to pass and are discharged through the reject stream, which goes to drain or can be fed back into the feed water supply in some situation to be recycled via the RO system to save water. The water that makes it through the RO membrane has around 95% to 99% of the dissolved salts eliminated from it.
It is vital to understand that a reverse osmosis system appoints cross filtration instead of standard filtration where the contaminants are gathered within the filter media. With cross filtration, the solution passes via the filter, or crosses the filter, with two outlets: the contaminated water goes one way and the filtered water goes a different way. To prevent accumulation of contaminants, cross flow filtration enables water to clear away contaminant build up and also permit enough commotion to keep the membrane surface dirt free.
Reverse Osmosis is very efficient in treating surface, ground and brackish water for both large and small flows applications and thus are commonly used in offices, homes and industrial sectors.